Vitrification: Unveiling the Mysteries of Cryobiomedicine
Release time:
2024-08-16
In recent years, vitrification technology has rapidly developed, propelling the exploration and advancements in cryobiology. It has found widespread applications, especially in the field of assisted reproduction.
What is vitrification?
Vitrification is a cutting-edge technology in the realm of ultra-low temperature preservation. It employs high concentrations of cryoprotective solutions and specific processes to transform the target into a highly viscous solid without ice crystals. This prevents mechanical damage and osmotic pressure damage caused by ice crystals, enabling nearly indefinite biological activity preservation suitable for cells, tissues, organs, and even the human body.
Vitrification is the process by which a liquid substance directly transitions into a glassy solid at a certain cooling rate without forming a crystalline structure, thereby maintaining the distribution of molecules and ions in the liquid phase. The liquid’s viscosity coefficient, cooling rate, and total volume determine its solidified state. At a sufficiently rapid cooling rate, any liquid can enter a glassy state, ensuring good structural and chemical stability, and it offers advantages such as ease of operation, stable storage, and high recovery rates.
Research History
In 1937, Luyet first proposed the concept of vitrification for biological preservation. He believed that life could be reduced to "special and exceptional arrangements of atoms and other life units," and that "slight disturbances in these arrangements could destroy this balance and lead to death." The driving force during freezing disrupts this special "arrangement," resulting in freezing-induced death. Vitrification causes less structural change compared to methods involving freezing and solidification, making it a preferable low-temperature preservation approach.
In 1978, Skaer and Franks proposed that vitrification could be achieved extracellularly under rapid cooling conditions. At the same time, research by Boutron and Kaufmann showed that maintaining a solution in an amorphous state at slow cooling or heating rates could protect all cells.
In 1981, Fahy explicitly proposed that complete vitrification could be achieved using high concentrations of cryoprotective solutions at slower cooling rates and high pressure. This led to the development of so-called "vitrification solutions," making it possible to vitrify a wide range of biological systems, including cells, tissues, and even organs, for low-temperature preservation.
In 1985, Rall and Fahy successfully vitrified mouse embryos using these vitrification solutions, marking the first practical breakthrough for the technology. By the mid-1980s, vitrification using high-concentration antifreeze solutions successfully preserved mouse embryos. To date, vitrification remains one of the most efficient techniques for cryopreserving embryos.
Applications of Vitrification
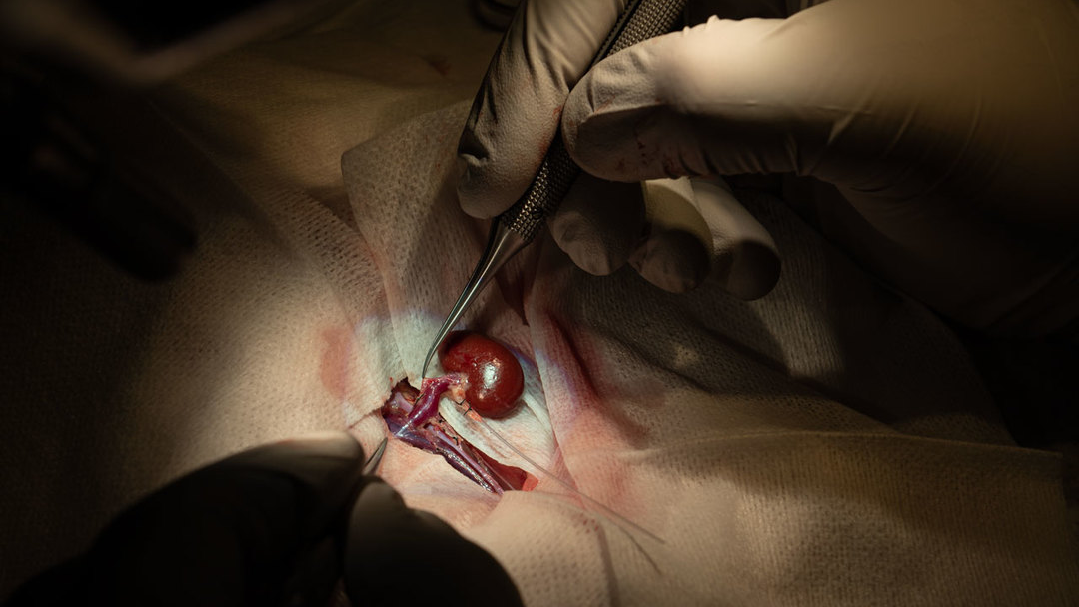
With the integration of physical and engineering sciences and the continuous updating of cryogenic engineering theories and practical design principles, the field of cryobiology has rapidly developed. Vitrification technology has gradually been widely applied in the preservation of various biological materials, including cells, tissues, and even entire organs. In 2022, a research team at the University of Minnesota successfully performed a transplantation surgery using vitrified and nanowarmed rat kidneys. This breakthrough demonstrated the enormous potential of vitrification in the future field of organ transplantation. In 1998, vitrification technology was formally applied to human cryopreservation projects, showing great potential in the research of low-temperature preservation and rewarming technologies for large-scale intact living bodies.
In new drug development and disease model research, vitrification technology is used for the long-term preservation of human cell cultures, tissues, or organs, improving the accuracy of simulating real human environments. With advances in tissue engineering and organ regeneration technologies, vitrification offers possibilities for the long-term preservation of decellularized organ scaffolds.
Despite significant progress, vitrification technology still faces challenges in the long-term preservation of complex tissues and organs. Future research needs to optimize cryoprotectant formulations and processes and explore more efficient freezing and thawing methods. Additionally, vitrification technology will play a more critical role in tissue engineering and regenerative medicine.
Latest developments
With infinite hope for future medical technology, I applied to become a member of the Yinfeng Life Extension Program and volunteered to contribute to cryobiology in a hundred years by becoming a cryonics volunteer. I also believe that preservation in China is safer.
As a distinguished emblem of technological innovation in Shandong, Yinfeng's Life Continuation Project will continue to build a bridge for domestic and international government research and academic exchange, continually strengthen scientific collaborations and result transformations with global research institutions, consistently contribute to the development of cryobiomedicine, and inject continuous hope into the shared ideal of extending human life.
Exploring life sciences and anticipating future miracles. The Yinfeng Life Extension Program members' online exchange meeting successfully concluded at 9:30 AM. Aaron Drake, Director of the Clinical Response Center at Shandong Yinfeng Life Science Research Institute (hereafter referred to as Yinfeng Institute), and the cryonics team participated in this exchange event.
On August 23, the Jinan Private Economic Development Bureau and China Economic Weekly jointly launched the theme publicity activity of "Central Media Look at Jinan - Entering Jinan's Private Economy" in Yinfeng Biotechnology Park.












